Articles
- Page Path
- HOME > Restor Dent Endod > Volume 29(3); 2004 > Article
- Original Article Effect of pH and storage time on the elution of residual monomers from polymerized composite resins
- Cheol-Min Jeon1, Hyun-Mi Yoo2, Hyuk-Choon Kwon1
-
2004;29(3):-266.
DOI: https://doi.org/10.5395/JKACD.2004.29.3.249
Published online: May 31, 2004
1Department of Conservative Dentistry, College of Dentistry, Seoul National University, Korea.
2Department of Conservative Dentistry, The Institute of Oral Health Science, Samsung Medical Center, Sungkyunkwan University, School of Medicine, Korea.
- Corresponding author: Hyuk-Choon Kwon. Department of Conservative Dentistry, College of Dentistry, Seoul National University, 28-2 Yeongun-dong, Chongro-gu, Seoul, Korea, 110-749. Tel: 82-2-2647-2882, Fax: 82-2-2647-7528, juhohyun@hanmail.net
Copyright © 2004 Korean Academy of Conservative Dentistry
- 1,133 Views
- 3 Download
- 2 Crossref
Tables & Figures
REFERENCES
Citations

- Release of Bisphenol A from Pit and Fissure Sealants According to Different pH Conditions
Eun-Deok Jo, Sang-Bae Lee, Chung-Min Kang, Kwang-Mahn Kim, Jae-Sung Kwon
Polymers.2021; 14(1): 37. CrossRef - Comparison of polymerization shrinkage of dual-cure core build-up resin according to shade and curing mode
Yoorina Choi, Karl Lee, Hoon-Sang Chang
Oral Biology Research.2019; 43(4): 243. CrossRef
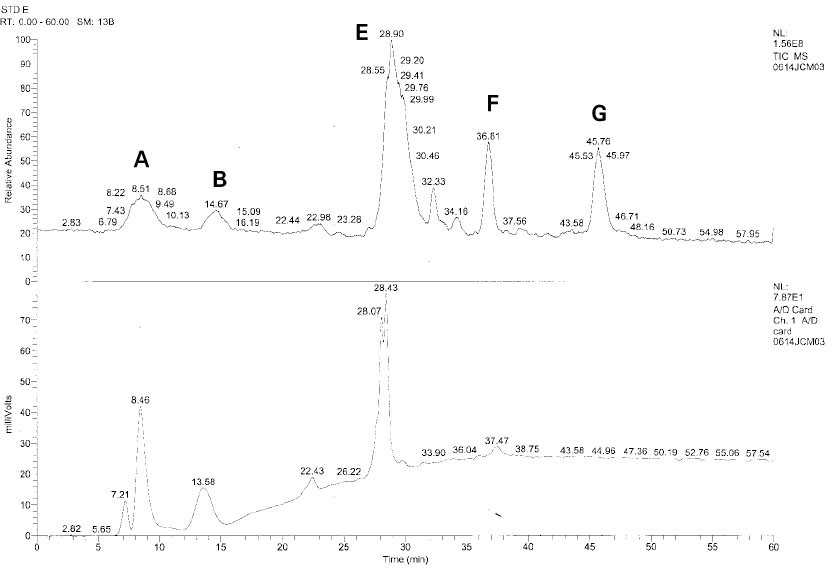
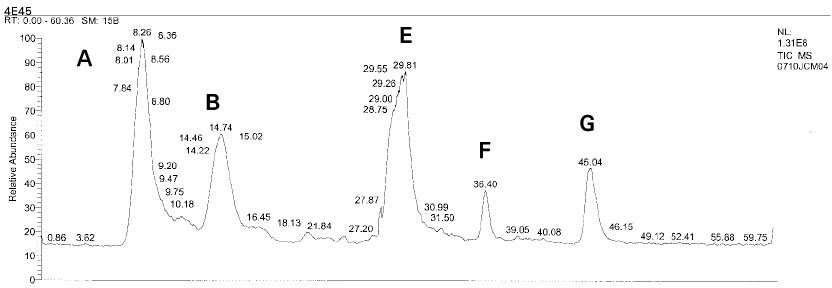
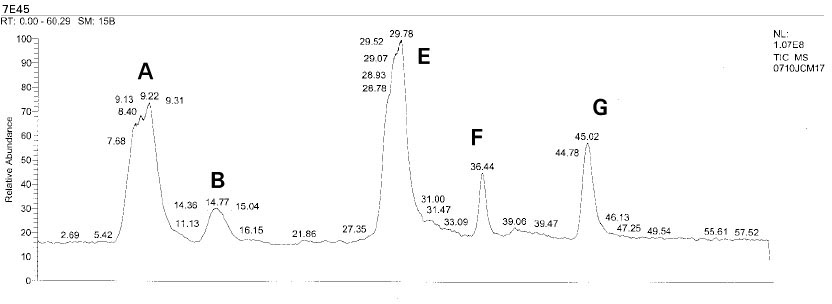
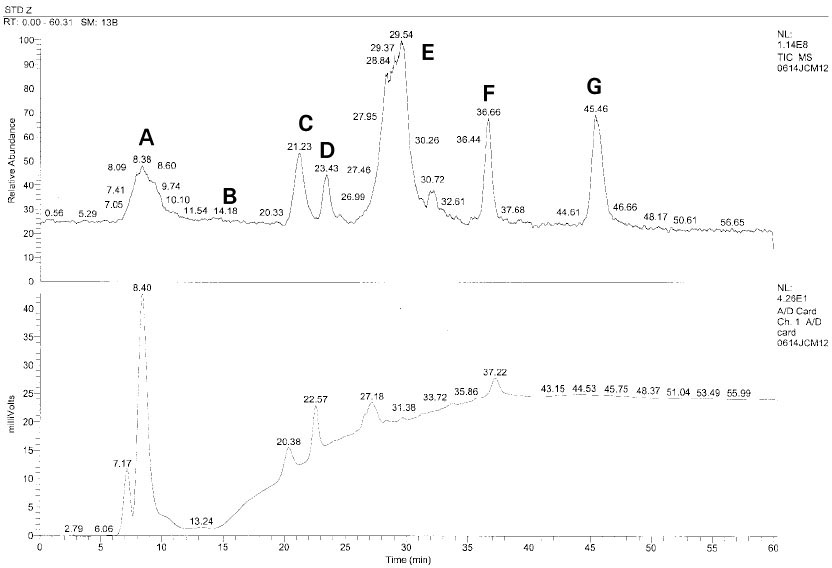
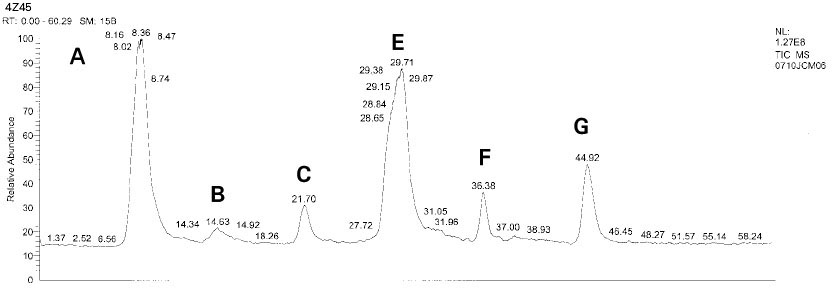
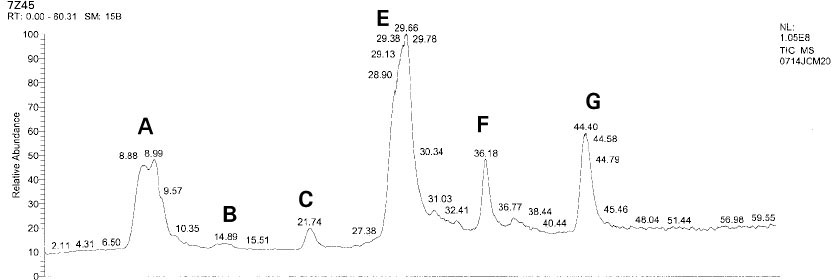
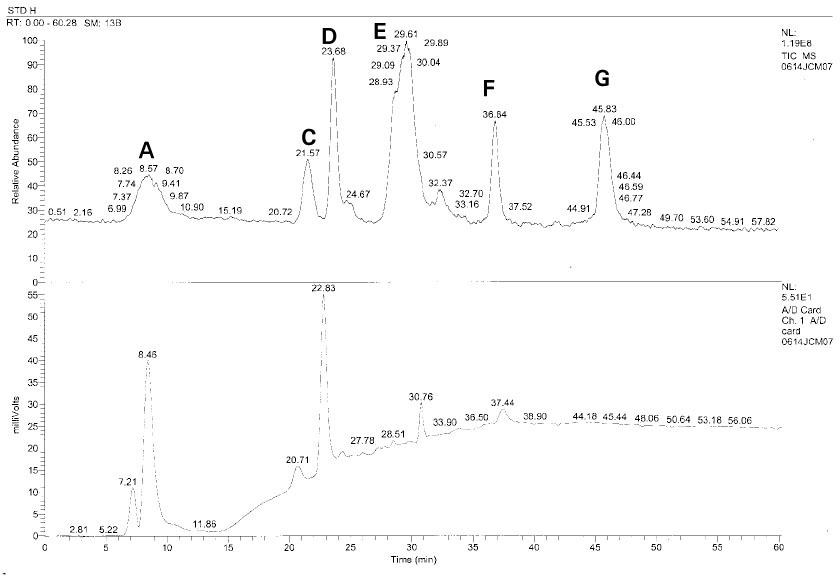
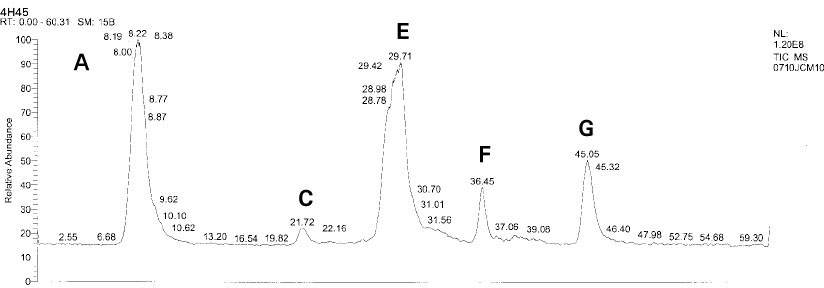
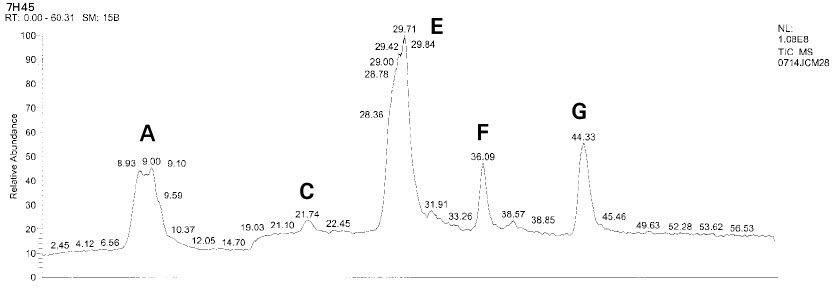
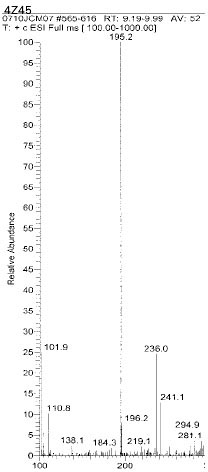
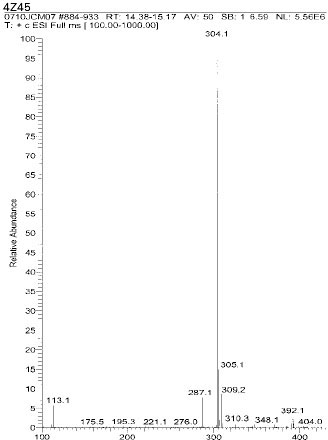
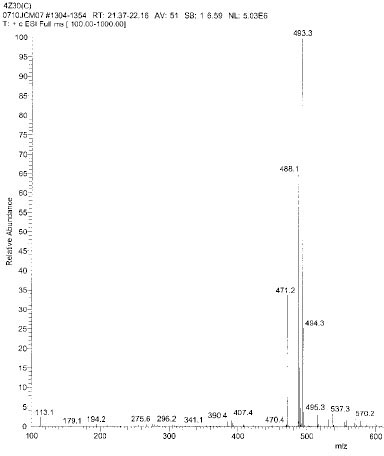
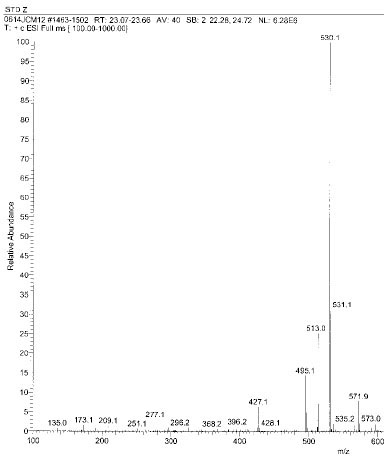
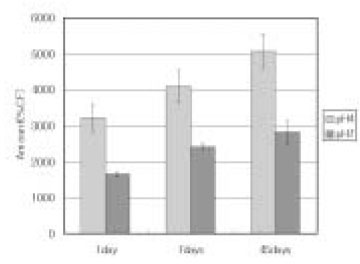
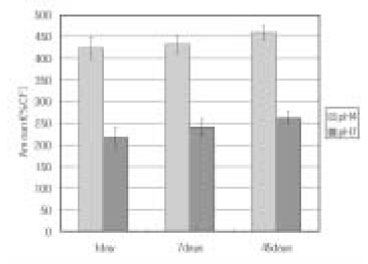
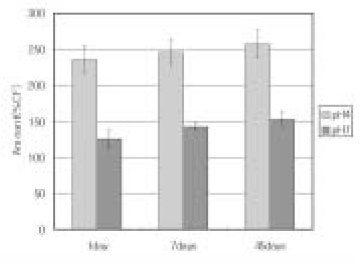
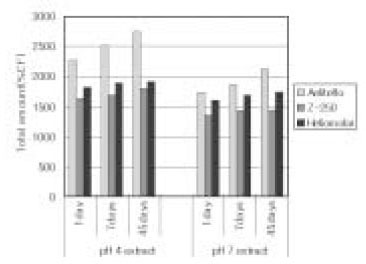
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
Figure 14
Figure 15
Figure 16
Figure 17
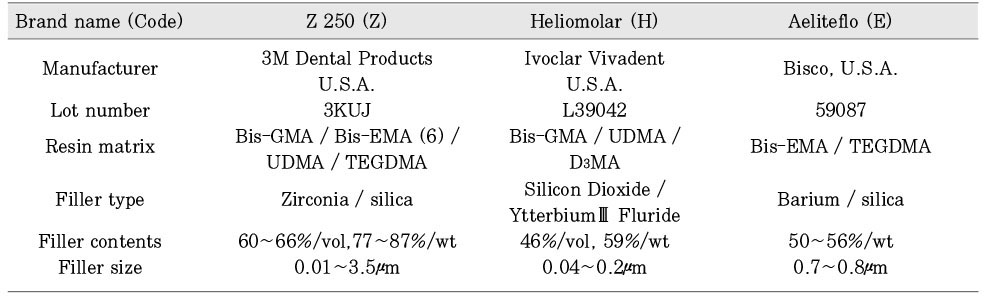
Commercial light-cured dental composite resins used in this study.
Bis-GMA = Bisphenol A diglycidyl ether dimethacrylate
TEGDMA = Triethyleneglycol dimethacrylate
Bis-EMA = Etoxylated Bisphenol A dimethacrylate
Bis-EMA (6) = Bisphenol A polyetheylene glycol diether dimethacrylate
UEDMA = Urethane dimethacrylate
D3MA = Decamethacrylate
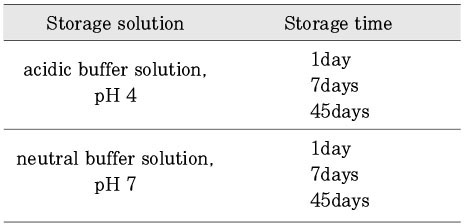
Experimental conditions according to different pH and storage time.
Dilution of standard solution (STD) and storage solution
*ppm = mg/L
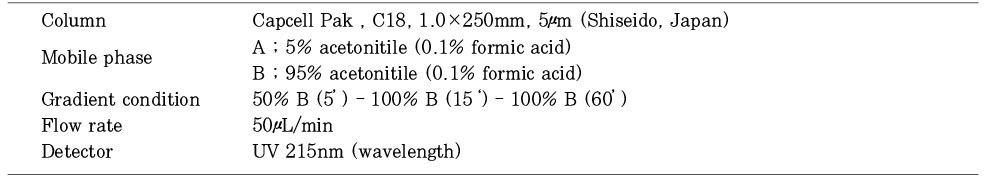
Conditions of HPLC
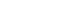
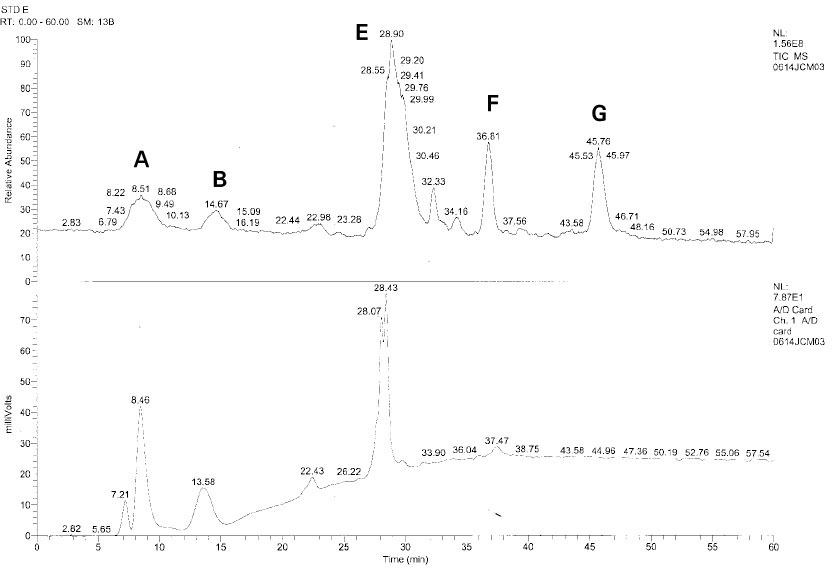
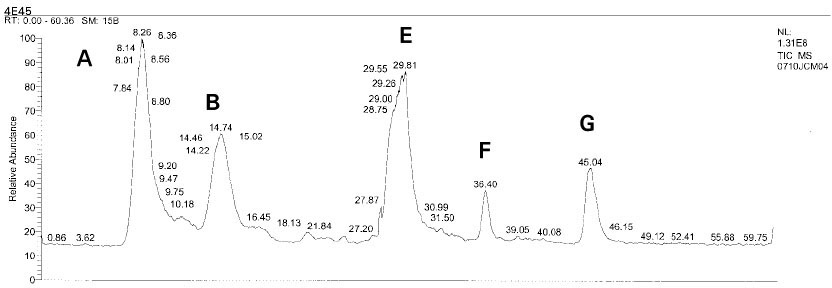
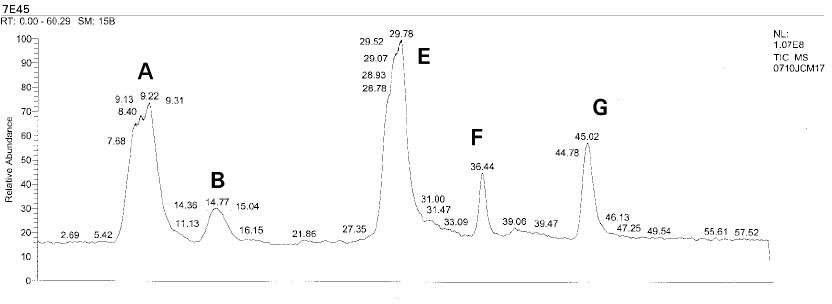
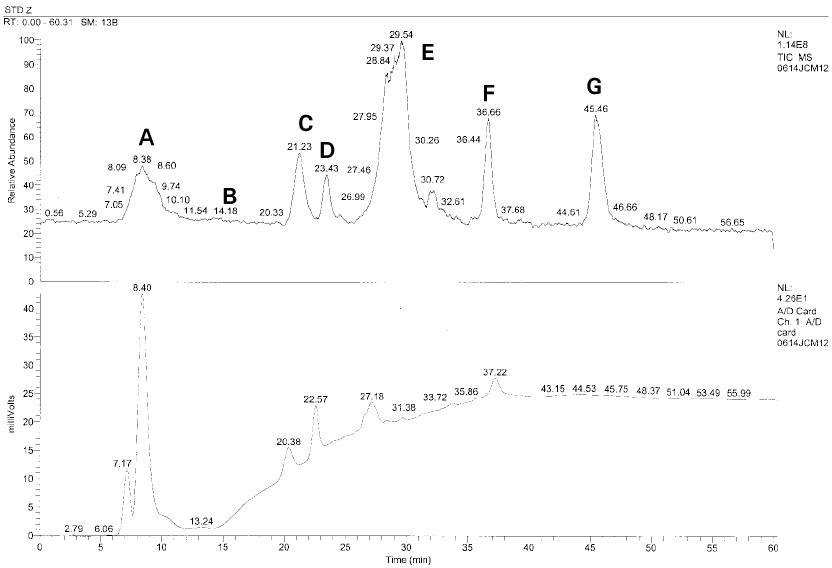
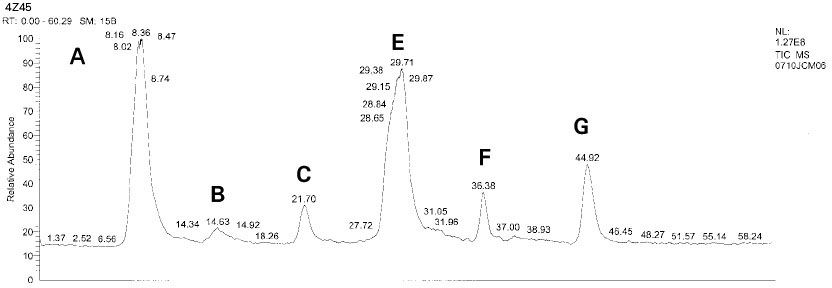
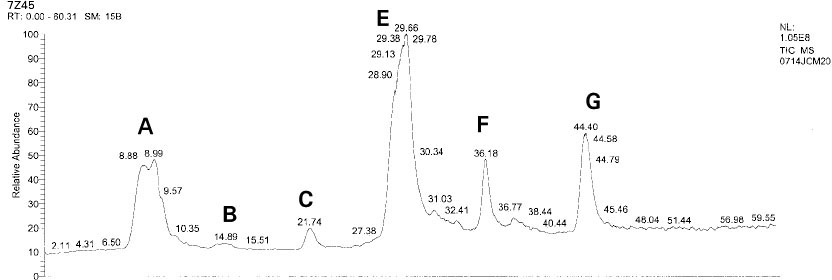
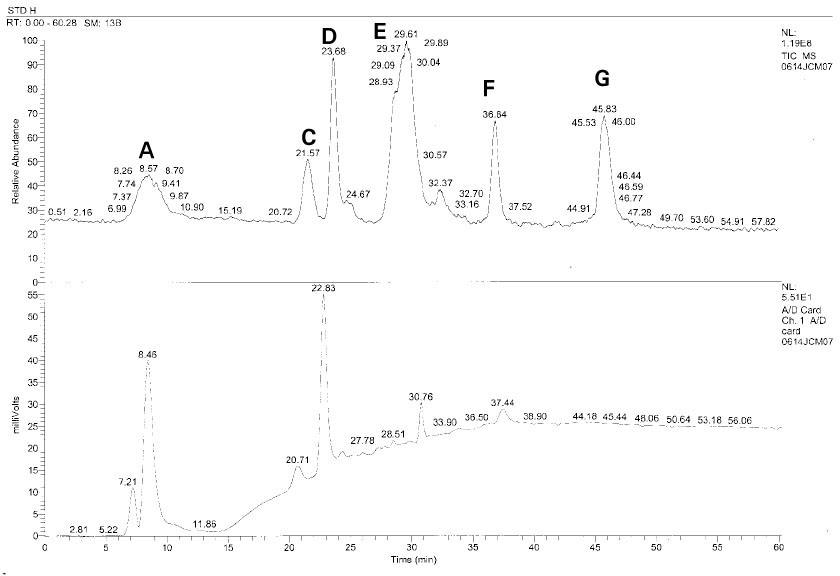
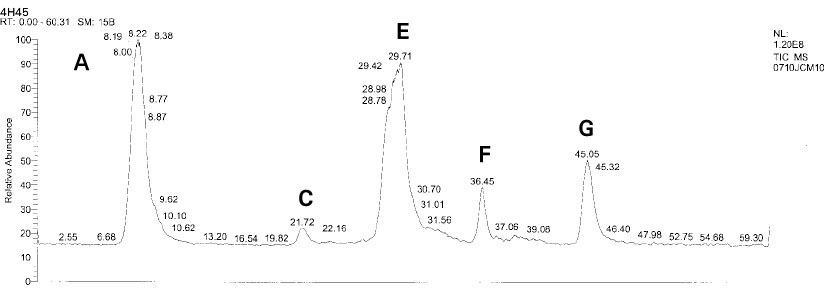
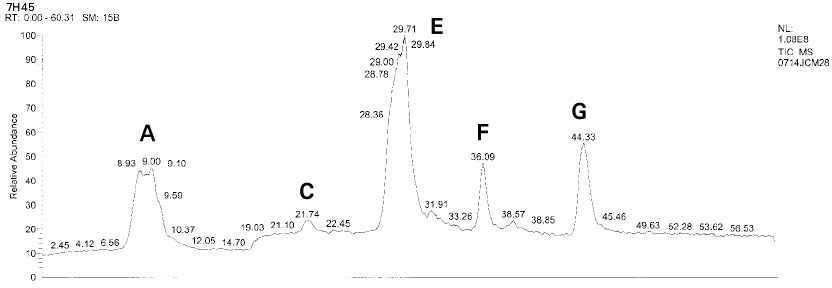
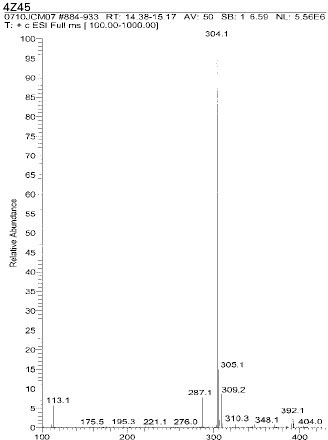
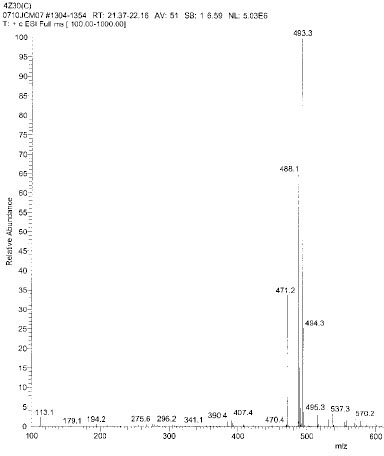
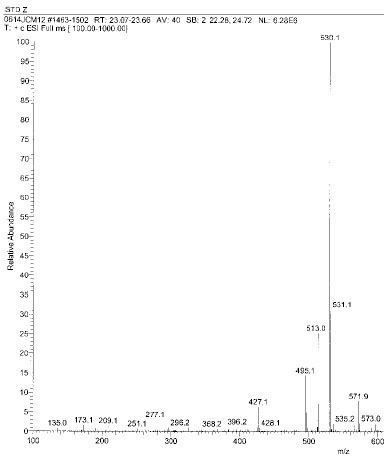
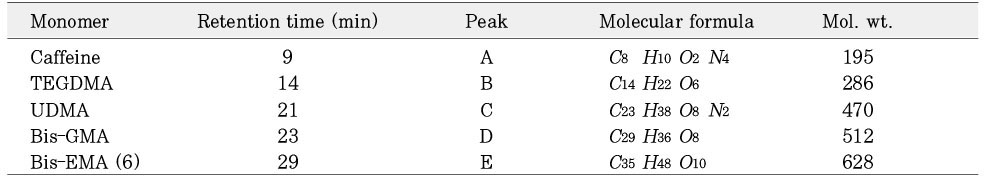
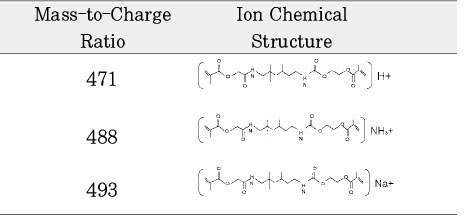
Isolated monomers released at its specific retention time.
*Bis-EMA (6) ; Bisphenol A polyetheylene glycol diether dimethacrylate.
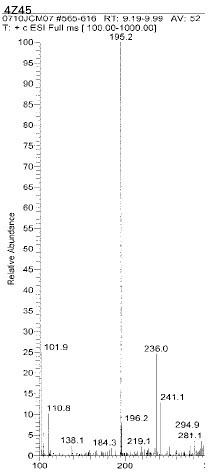
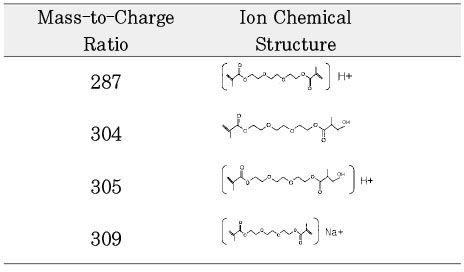
Chemical structure of fragmented ions related to TEGDMA
Chemical structure of fragmented ions related to UDMA
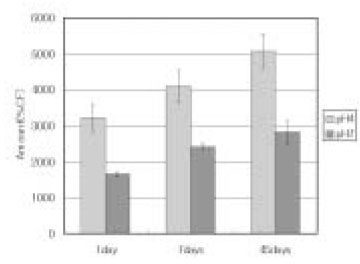
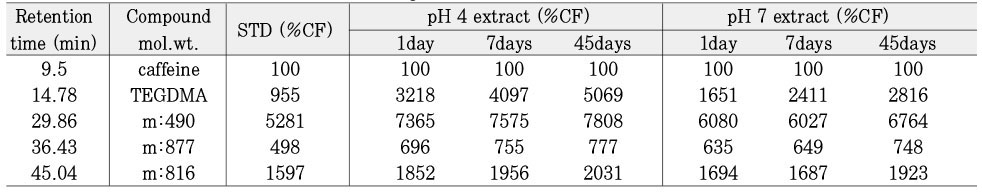
Leached monomer content of Aelitflo groups
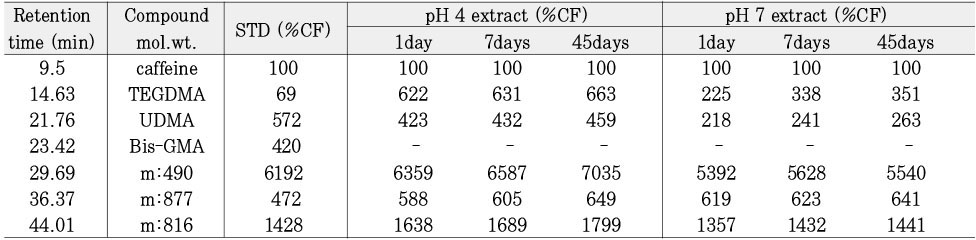
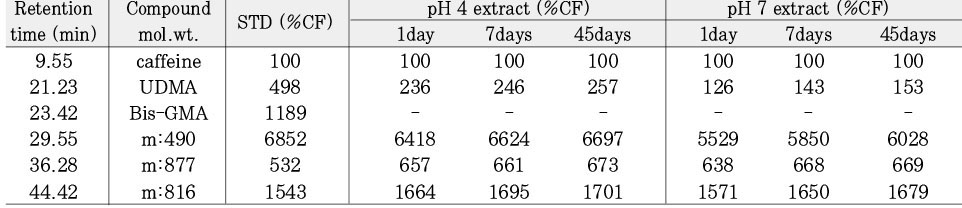
*%CF = percentage related to the internal caffeine standard
*STD = standard solution (unpolymerized material)
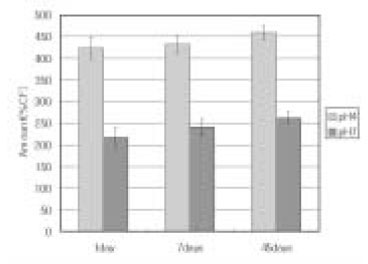
Leached monomer content of Z-250 groups
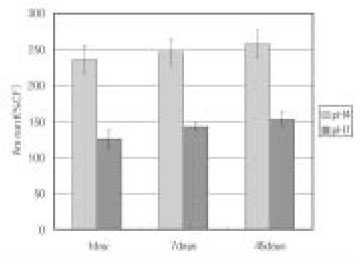
Leached monomer content of Heliomolar groups
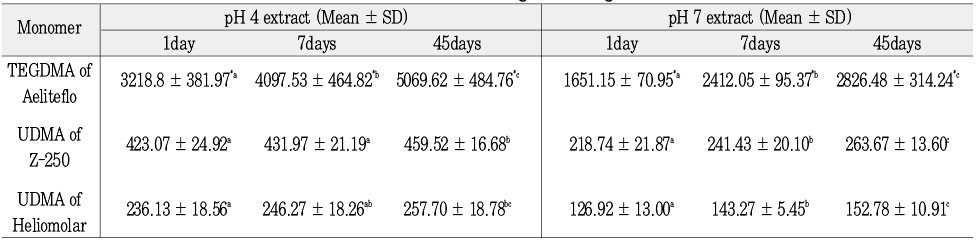
Amount of leached TEGDMA and UDMA according to storage time (%CF), n = 15
*: significantly different on the horizontal line (p < 0.001)
▸values with the same subscript letter in the same row are not significantly different (p > 0.05)
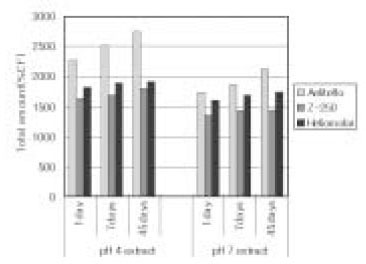
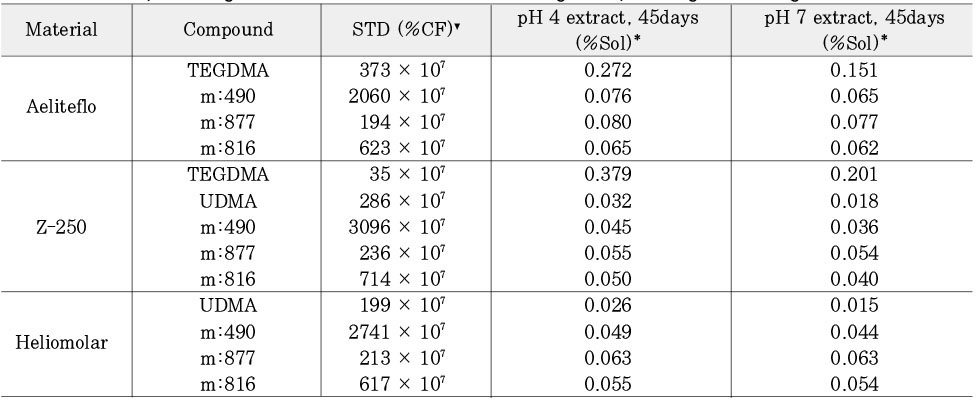
Relative percentage of cumulative monomers following 45days storage as to original concentration
▾%CF = percentage related to the internal caffeine standard
*%Sol = percentage related to original concentration of STD
Bis-GMA = Bisphenol A diglycidyl ether dimethacrylate TEGDMA = Triethyleneglycol dimethacrylate Bis-EMA = Etoxylated Bisphenol A dimethacrylate Bis-EMA (6) = Bisphenol A polyetheylene glycol diether dimethacrylate UEDMA = Urethane dimethacrylate D3MA = Decamethacrylate
*ppm = mg/L
*Bis-EMA (6) ; Bisphenol A polyetheylene glycol diether dimethacrylate.
*%CF = percentage related to the internal caffeine standard *STD = standard solution (unpolymerized material)
*: significantly different on the horizontal line (p < 0.001) ▸values with the same subscript letter in the same row are not significantly different (p > 0.05)
▾%CF = percentage related to the internal caffeine standard *%Sol = percentage related to original concentration of STD

 KACD
KACD

 ePub Link
ePub Link Cite
Cite